AGS Therapeutics Unveils Ambitious R&D Pipeline, Showcasing the Potential of its Universal Delivery System for Novel Therapeutics
The company’s MEV universal delivery system has the potential to overcome biological barriers in the body and solve current drug delivery challenges.
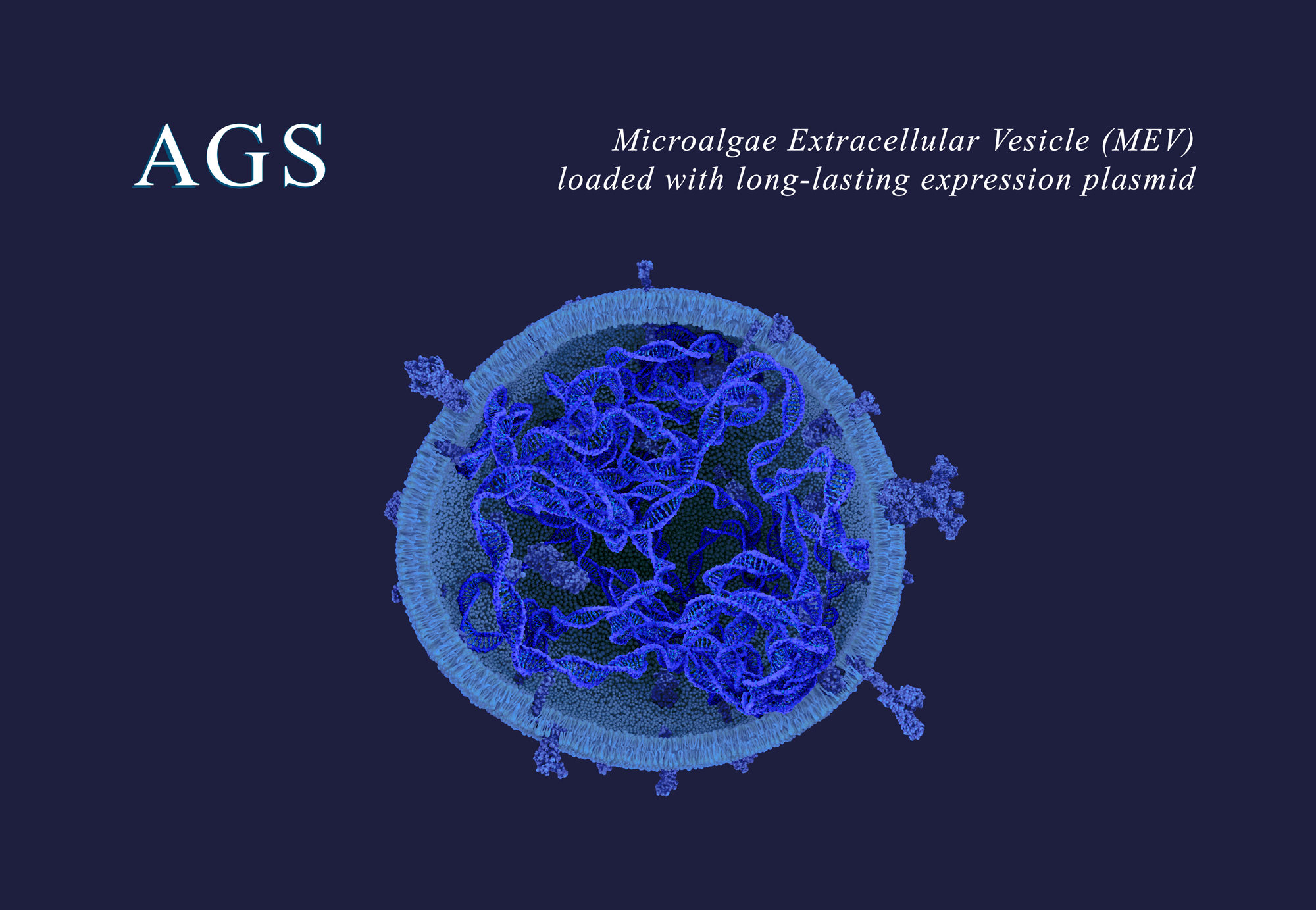
Paris, April 3, 2023 – AGS Therapeutics, an early-stage biotech company pioneering micro-algae extracellular vesicles (MEV) as a new delivery system for innovative therapeutic treatments, today announced its initial internal research and development (R&D) pipeline. It is designed to showcase the potential of its MEVs as a new groundbreaking delivery system for bringing a wide range of payloads, including mRNA, siRNA, plasmid-DNA, antisense oligonucleotides (ASO), peptides, and proteins, to a variety of tissues and organs for treatment of a wide range of diseases. MEVs can overcome many of the challenges met by current delivery systems based on mammalian extracellular vesicles and on synthetic lipid nanoparticles.
“AGS' internal R&D pipeline reflects the competitive advantage and differentiation of our MEVs from current delivery systems as well as MEVs’ natural ability to target specific organs and tissues”, said AGS CEO Manuel Vega. “It represents the broad potential of our technology as we advance towards preclinical and clinical development.”
AGS initial internal R&D pipeline focuses on a few key areas where MEVs’ unique capabilities could make a significant impact in the life of patients with critical, unmet medical needs. These areas include eye drops for retinal diseases (AGS-1010), oral treatments for diseases of the intestinal epithelium (AGS-2010 and AGS-2020) and inhaled treatments for pulmonary diseases and their related residual infections (AGS-3010 and AGS-3020).

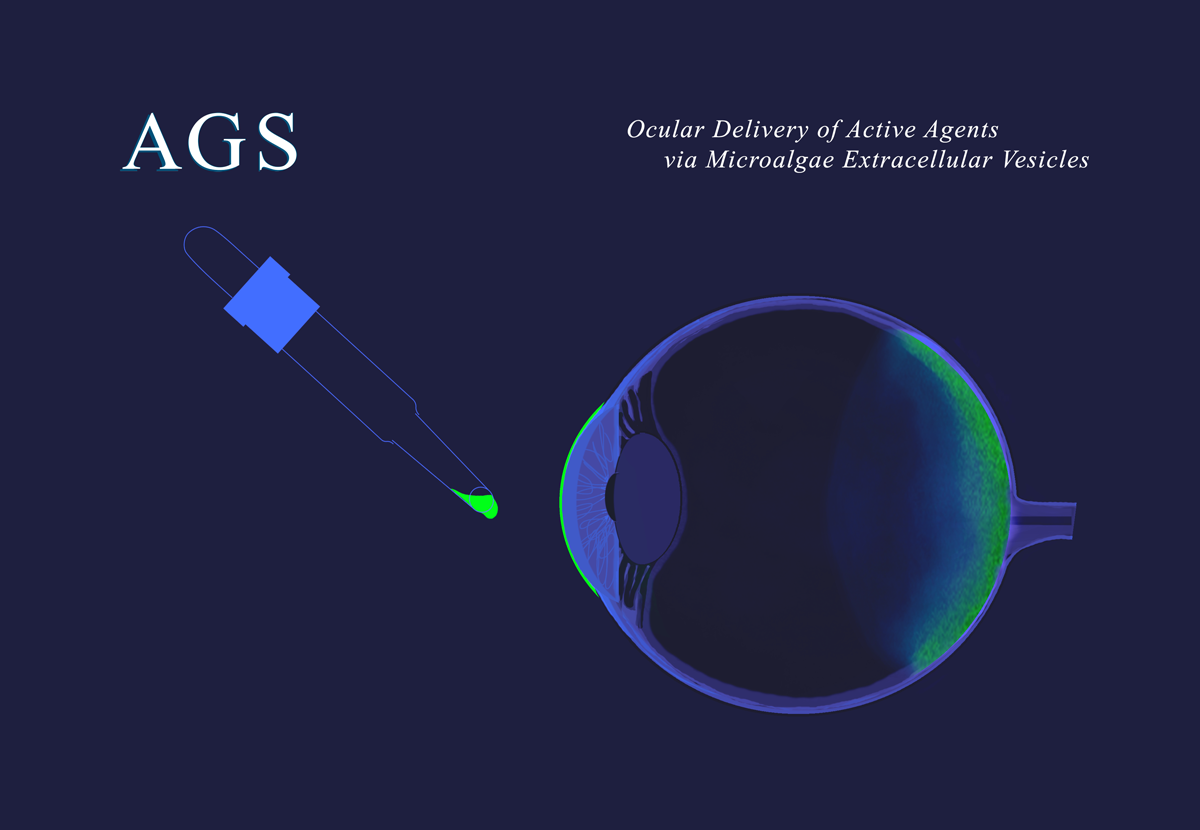
1. Eye drops for Retinal diseases (AGS-1010)
Despite the advances in treating retinal diseases, such as wet age-related macular degeneration (wAMD), many patients either don’t receive treatment or cease their intravitreal therapies too early, compromising clinical outcomes. Retrospective studies have shown that up to 35% of patients definitively stop their treatment after only six months, putting themselves at risk of becoming blind.
Simple eye drops of AGS' MEVs have been shown in vivo to reach the choroid-retina tissue and to deliver active payloads. AGS hopes to bring a new, more patient-friendly treatment modality to patients suffering from wAMD disease and expand access to therapy.
Current therapies against retinal diseases consist of intravitreal injections (injections inside the eyeball) of anti-angiogenic agents administered regularly. Global sales of these therapies in 2022 were about $15 billion, with nearly two-thirds in wAMD.
2. Oral treatments for diseases of the Intestinal Epithelium (AGS-2010 and AGS-2020)
AGS’ oral treatments for inflammatory, immune or metabolic diseases of the intestinal epithelium leverage the ability of MEVs to safely transport biologics through the harsh environment of the stomach into the epithelium in the lining of the intestine. New modalities such as RNA-based therapies are promising treatments for disorders involving the intestinal epithelium provided they can be delivered to the proper tissue. The market for current treatments of inflammatory and immune diseases of the epithelium is several billion U.S. dollars globally.
3. Inhaled treatments for Pulmonary diseases (AGS-3010 and AGS-3020)
In vivo AGS’ MEVs have shown that when inhaled, they are able to reach the pulmonary epithelium and deliver payloads that are expressed in the lung cells. By leveraging MEV’s natural function of delivering messages, MEV-3010 and MEV-3020 are designed to address rare pulmonary genetic diseases and their related inflammation and residual infections, respectively.
“The unveiling of AGS’ initial internal R&D pipeline marks a key strategic step in showing the versatility of our breakthrough universal delivery system, which can bring innovative payloads of different types to various tissues and organs depending on the route of administration,” said AGS Chief Corporate Development Marie-Hélène Leopold. “We also intend to setup an external pipeline with collaborators to fully explore the potentiality of our technology, like for instance in CNS or neurodegenerative diseases as our MEVs have been shown to reach different brain regions without having to cross the blood-brain barrier. The truly unique capabilities of MEVs have the potential to revolutionize targeted drug delivery in many therapeutic areas with high medical needs."
Several promising projects in AGS’ R&D pipeline are set to enter preclinical and clinical trials within the next two years. Investigational new drug (IND) applications for the first two projects are expected to be submitted in the U.S. in the second half of 2025. By leveraging its breakthrough delivery system, AGS Therapeutics believes it can develop life-changing therapies and technologies that may have a transformative impact on the lives of patients worldwide.
About AGS
AGS Therapeutics, based in Paris, France, is a biotech company pioneering the development of biomedicines based on extracellular vesicles from microalgae (MEV) that have been shown to be a safe, targeted and highly versatile delivery system for innovative biologics, such as mRNA, siRNA, DNA, plasmids, and proteins for a broad range of human diseases. AGS-M, the company’s subsidiary and a contract development and manufacturing organization, will produce the MEV needed to support preclinical and clinical development of MEV-based product pipelines from AGS and from pharmaceutical companies partnering with AGS. AGS’ MEVs are derived from Chlorella, a two-billion-year-old single-cell algae used for decades as a food supplement. AGS’ MEVs are easy to manufacture in large quantities with simple cell culture media production techniques that are both eco-friendly and easily scalable. Through strategic partnerships and a commitment to scientific excellence, the company aims to revolutionize the healthcare landscape and improve the lives of patients across the globe. For more information visit www.ags-tx.com and www.ags-m.com.
Forward looking statements
This announcement may include predictions, estimates or other information that might be considered forward-looking. While these forward-looking statements represent our current judgment on what the future holds, they are subject to risks and uncertainties that could cause actual results to differ materially. You are cautioned not to place undue reliance on these forward-looking statements, which reflect our opinions only as of the date of this communication.
Contacts - US:
Dan Eramian | Opus Biotech Communications | +1 425-306-8716 | danieleramian@comcast.net
Charles Craig | Opus Biotech Communications | +1 404-245-0591 | charles.s.craig@gmail.com
Contacts - Europe:
Marie-Hélène Leopold | AGS Therapeutics | +33 (0)6 07 16 55 01 | mhl@ags-tx.com
Ana Vega | Markets & Listing | +33 (0)6 88 57 05 77 | av@markets-listing.com