AGS Therapeutics Strengthens its Patent Portfolio on Microalgae Extracellular Vesicles (MEVs) with an International Patent Application on the use of MEVs to deliver biological agents to the brain by the intranasal route of administration.
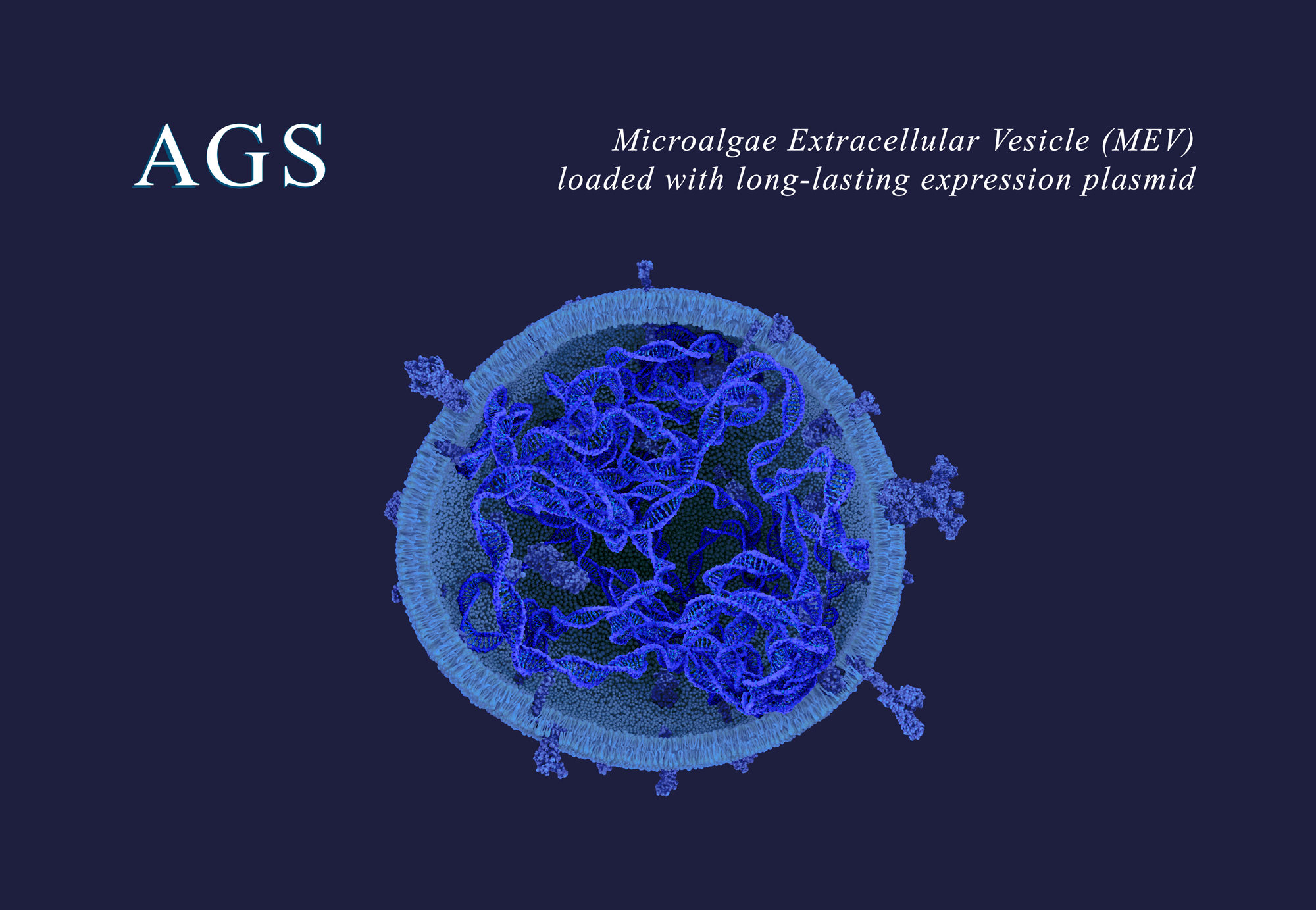
MEVs are a universal delivery system; suitable for delivering innovative therapeutics, vaccines and gene therapies. MEVs can be administered through multiple routes; they overcome stringent biological barriers and reach difficult-to-access tissues, providing the potential to overcome challenges faced by other delivery systems. MEVs deliver their payload in target tissues of interest, where the payload elicits its desired biological activity. MEVs can be loaded with a diversity of payloads or therapeutic modalities.
PARIS, FRANCE, May 7, 2024 – AGS Therapeutics, a preclinical-stage biotech company pioneering microalgae extracellular vesicles (MEV) as a new, universal delivery system, announced the recent publication of its PCT International Application No. PCT/EP2023/078634 – Publication - WO 2024/088808, titled Extracellular Vesicles from Microalgae, their Biodistribution upon Intranasal Administration, and Uses Thereof.

Thanks to their rare and natural capacity to overcome certain stringent biological barriers, MEVs can be used to deliver payloads to tissues and organs which have been so far either difficult or impossible to access by current alternative systems like LNPs, viral gene therapy vectors, or mammalian EVs. The blood-brain-barrier (BBB), a natural obstacle that protects the brain from agents arriving from the blood, is a canonical example of such stringent barriers. So much so, that efforts to overcome the BBB have consumed focus and large amounts of ressources for decades, and continue doing so today.
Upon intranasal administration, MEVs are internalized by olfactory sensory neurons (OSNs) present at the bottom of the intranasal epithelium. OSNs are primary neurons which are, literally, projections from the brain onto the nose. Thus, when MEVs are internalized by OSNs, they enter the brain, directly. Once inside neurons, MEVs travel intracellularly by axonal transport and jump over synapsis to surrounding neurons in the network. In doing so, MEVs are able to travel throughout the lateral olfactory tracts to reach specific regions of the brain, including the cortex as well as the limbic brain. Moreover, agents released by MEVs are released inside the cell, in contrast to agents eventually arriving from the blood. As such, MEVs are always “on the brain side” and the BBB is an irrelevant biological barrier in the delivery of therapeutic modalities using the MEVs as delivery system.
“The published patent application highlights the potential to use MEVs for the delivery of biological agents to specific regions of the brain, by simple intranasal administration. According to Mondor Intelligence, the global market for CNS Therapeutics is expected to grow at a CAGR of 6% from $113 billion in 2023 to $151 billion in 2028, driven by the patients' demography for CNS diseases and innovations. New delivery systems are needed for these devastating diseases, and we believe that MEVs could play a critical role as a new delivery system for innovative CNS therapeutics from diverse modalities”, said Marie-Helene Leopold, Chief Corporate Development Officer, AGS Therapeutics.
AGS’ portfolio of Intellectual Property is managed by Stephanie Seidman, at Womble Bond Dickinson, San Diego (www.womblebonddickinson.com/us/people/stephanie-seidman).
About AGS
AGS Therapeutics, based in Paris, France, is a biotech company pioneering the use of extracellular vesicles from microalgae (MEVs) as a universal delivery system for innovative biologics and gene therapies. AGS has shown MEVs to be a safe, targeted and highly versatile delivery system for mRNA, siRNA, DNA oligos, plasmids, proteins, and peptides relevant to a broad range of human diseases. AGS-M, a company’s CDMO subsidiary, produces the MEVs needed to support R&D and product development from AGS and from companies partnering with AGS. AGS’ MEVs are derived from Chlorella, a two-billion-year-old single-cell algae, labelled by FDA as GRAS for consumption as a food suplement. AGS’ MEVs are easy to manufacture in large quantities with processes that are both eco-friendly and easily scalable. Through strategic partnerships and a commitment to scientific excellence, the company aims to challenge the delivery landscape and improve the lives of patients across the globe. For more information visit www.ags-tx.com and www.ags-m.com.
Forward looking statement
This announcement may include predictions, estimates or other information that might be considered forward-looking. While these forward-looking statements represent our current judgment on what the future holds, they are subject to risks and uncertainties that could cause actual results to differ materially. You are cautioned not to place undue reliance on these forward-looking statements, which reflect our opinions only as of the date of this communication.
Contacts
Marie-Hélène Leopold | AGS Therapeutics | +33 (0)6 07 16 55 01 | mhl@ags-tx.com
Ana Vega | Markets & Listing | +33 (0)6 88 57 05 77 | av@markets-listing.com