Non-Invasive Back-of-the-Eye Treatment: AGS Therapeutics Strengthens Its Patent Portfolio with International Patent Application for MEV-Based Ocular Delivery
Published patent application highlights the use of MEVs to deliver therapies non-invasively to the back of the eye, providing treatments for conditions such as macular degeneration. Topical administration of MEVs could transform treatment options for millions of patients worldwide. Patent application supports the company’s AGS-1010 program, targeting multi-billion-dollar markets in eye disease therapies.
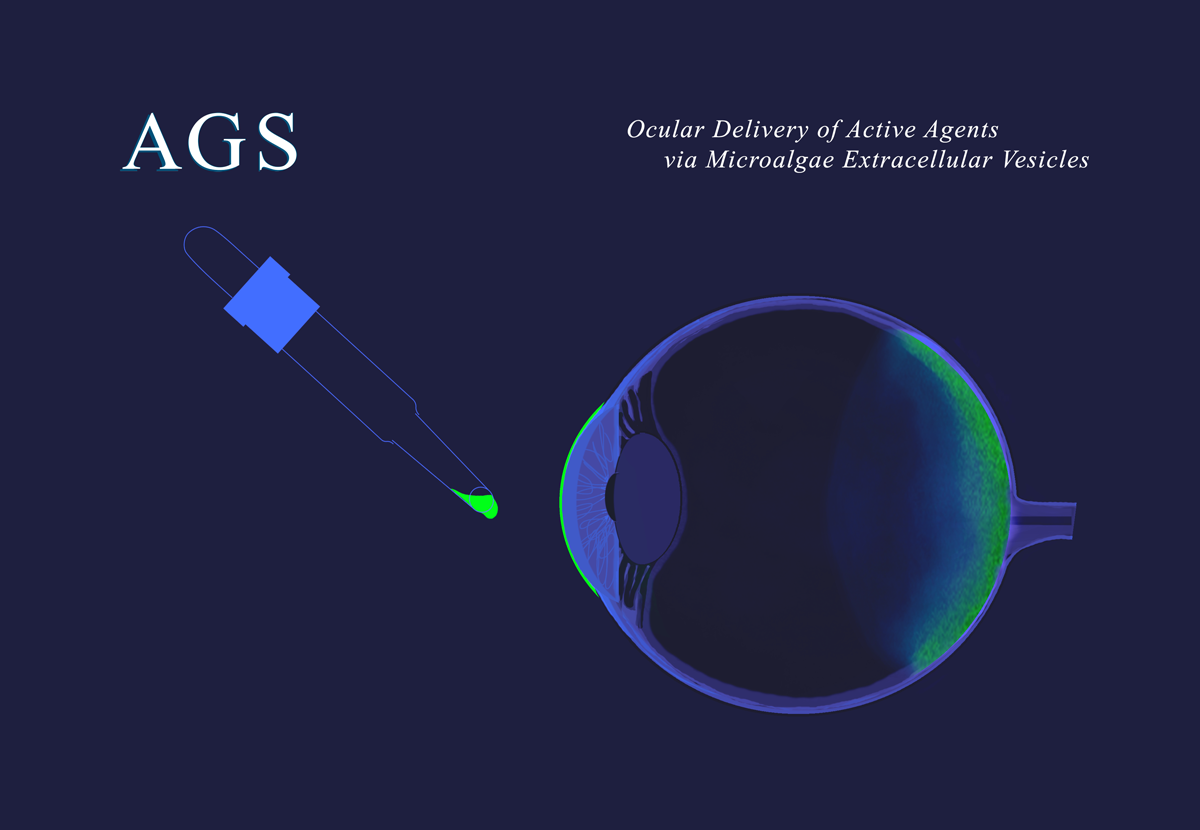
Evry, France, September 5, 2025 – AGS Therapeutics, a preclinical-stage biotech company pioneering microalgae extracellular vesicles (MEVs) as a new, universal delivery system, announced the recent publication of its PCT International Application N° PCT/EP2025/054728 - Publication WO2025/176847, titled Ocular Delivery of Active Agents via Microalgae Extracellular Vesicles.
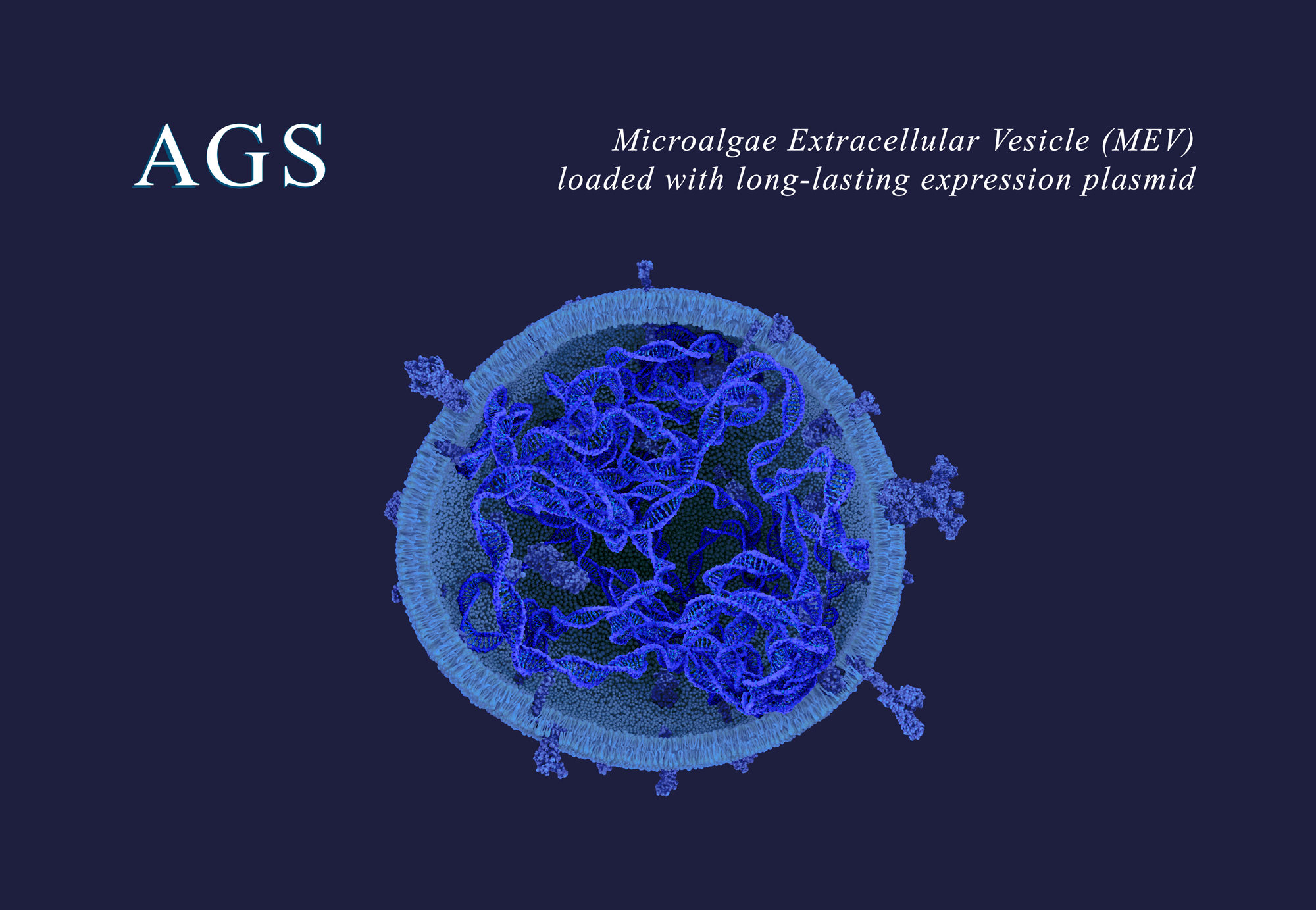
MEVs represent a universal delivery system suitable for innovative therapeutics, vaccines, and gene therapies. They can be loaded with a wide range of payloads and administered through multiple routes. By overcoming stringent biological barriers and reaching hard-to-access tissues, MEVs address challenges that have limited other delivery platforms. Thanks to their rare and natural capacity to cross stringent biological barriers, MEVs enable the delivery of therapeutic payloads to tissues and organs that are difficult—or until now, impossible—to reach with conventional systems such as lipid nanoparticles (LNPs), viral gene therapy vectors, or mammalian EVs. Once delivered to target tissues, MEVs release their payload, triggering the intended biological activity.
“ Our research demonstrates that, upon topical ocular administration of loaded MEVs, they can travel across the eye, reach the choroid and retina, and deliver biologically active payloads such as mRNA to those cells,” said Lila Drittanti, AGS’ Chief Operating Officer.“This unprecedented ability opens the door to non-invasive treatments for serious eye conditions that today require invasive procedures.”
A notable example is MEV-mediated delivery of mRNA to the back of the eye—specifically to choroid and retinal cells (including RPE, rods, and cones)—following simple topical instillation on the eye surface, which is expected to be suitable for the treatment of disorders related to neoangiogenesis like wAMD, as well as of metabolic or genetics diseases of the eye.
“The publication of this patent application is a foundational milestone for AGS. It demonstrates our leadership in the MEV field and strengthens the intellectual property behind our AGS-1010 product family, which targets large and growing ophthalmology markets worth billions of dollars worldwide,” said Marie-Helene Leopold, AGS’ Chief Corporate Development Officer.
“The potential impact is both scientific and societal,” added Manuel Vega, AGS’ CEO. “Topical administration—a route not feasible with alternative delivery systems—represents a major step forward for patient compliance, safety, and accessibility, always under the control of qualified ophthalmic practitionners. We believe MEVs could become a game-changing technology in the treatment of macular degeneration and other back-of-the-eye diseases.”
AGS’ portfolio of Intellectual Property is managed by Stephanie Seidman, at Womble Bond Dickinson, San Diego (www.womblebonddickinson.com/us/people/stephanie-seidman).
About AGS
AGS Therapeutics (Evry, France) is pioneering the use of microalgae extracellular vesicles (MEVs) as a universal delivery system for innovative therapeutics, vaccines, and gene therapies. AGS has demonstrated that MEVs are a safe, targeted, and highly versatile delivery platform for mRNA, siRNA, DNA oligonucleotides, plasmids, proteins, and peptides relevant to a wide range of human diseases. AGS-M, the company’s CDMO subsidiary, manufactures MEVs to support AGS’s own research as well as collaborative programs with partner companies. The company’s MEVs are derived from Chlorella, a two-billion-year-old single-cell alga classified by the FDA as GRAS (Generally Recognized as Safe) for consumption as a food supplement. MEVs can be manufactured at scale through eco-friendly and cost-efficient processes. Through strategic collaborations and a commitment to scientific excellence, AGS aims to transform the drug delivery landscape and improve patient outcomes worldwide. For more information, visit www.ags-tx.com and www.ags-m.com.
Forward looking statement
This announcement may include predictions, estimates or other information that might be considered forward-looking. While these forward-looking statements represent our current judgment on what the future holds, they are subject to risks and uncertainties that could cause actual results to differ materially. You are cautioned not to place undue reliance on these forward-looking statements, which reflect our opinions only as of the date of this communication.
Contacts
Marie-Hélène Leopold | AGS Therapeutics | +33 (0)6 07 16 55 01 | mhl@ags-tx.com
Ana Vega | Markets & Listing | +33 (0)6 88 57 05 77 | av@markets-listing.com