AGS Advances MEV Manufacturing to Clinical-Grade GMP through Shared Manufacturing Organization Agreement with INITS SMO
A milestone collaboration securing access to clinical-grade GMP manufacturing of MEVs and accelerating clinical readiness for AGS’ lead programs.
Évry-Courcouronnes, France — February 16, 2026. AGS Therapeutics (AGS), a biotechnology company pioneering microalgae-derived extracellular vesicles (MEVs) as a next-generation drug delivery platform, today announced the signing of an agreement with INITS SMO, a GMP-qualified Shared Manufacturing Organization (SMO), to support the transition of its MEV manufacturing activities to full GMP compliance and enable the production of its first clinical-grade MEV batches.
Under the agreement, AGS will operate its proprietary MEV manufacturing, loading, and analytical processes within INITS’ GMP-qualified infrastructure. INITS SMO provides access to facilities, equipment, and an established quality system compliant with GMP requirements and recognized by both European regulatory authorities and the U.S. Food and Drug Administration.. Throughout the collaboration, AGS retains full ownership and operational control of its technology, processes, equipment, intellectual property, and teams.
The collaboration is structured explicitly as a Shared Manufacturing Organization model, distinct from a contract development and manufacturing arrangement. No technology transfer is performed, and no third party takes operational ownership of AGS’s processes. Instead, AGS accesses GMP-qualified infrastructure, shared equipment, and quality system framing, while remaining fully responsible for execution and performance.
This agreement represents a deliberate and transitional operational step. It enables AGS to reach GMP maturity and key manufacturing milestones in parallel with the continued preparation of its own independent, modular GMP Demonstration Manufacturing Unit. Importantly, this approach enables progress toward clinical manufacturing without committing at this stage to a permanent site, long-term manufacturing partner, or fixed industrial footprint.
By combining speed of execution with capital discipline, the SMO framework significantly reduces risk in early clinical manufacturing while preserving strategic flexibility. It provides AGS with a controlled, inspection-ready environment to validate GMP processes, train teams, and consolidate industrial know-how, while maintaining full optionality for future scale-up, internalization, or alternative manufacturing configurations.
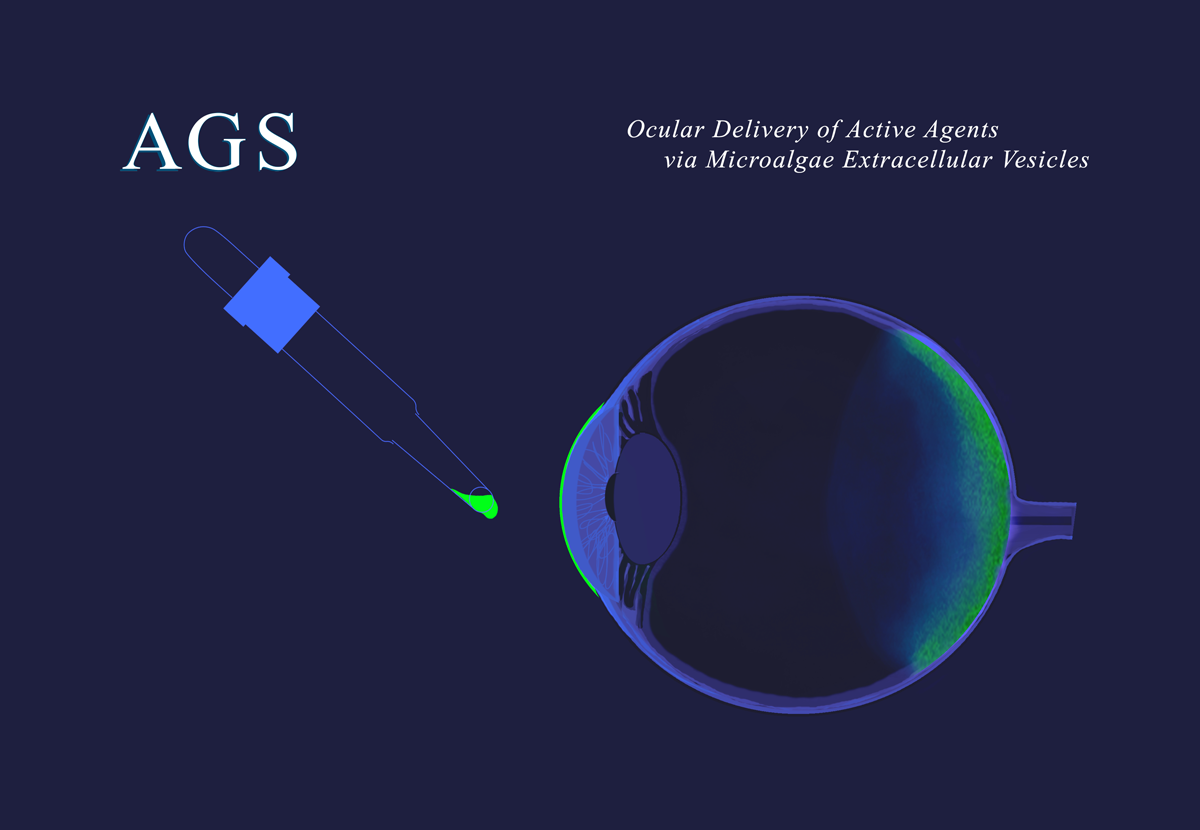
Through this agreement, AGS plans to manufacture GMP clinical batches for its two lead internal programs: AGS-1010 for wet age-related macular degeneration and AGS-2010 for inflammatory bowel disease, with GMP batch availability targeted for the end of 2027. In addition, the collaboration establishes a foundation for future GMP manufacturing activities supporting pharmaceutical, vaccine, and cosmetic partners developing MEV-based products.
“This agreement with INITS SMO marks an important execution milestone for AGS. It allows us to secure GMP readiness and advance toward clinical manufacturing while fully preserving ownership, control, and long-term strategic value. The SMO model gives us the right balance between operational rigor, capital efficiency, and independence.”
— Marie-Hélène Leopold, Chief Corporate Development Officer, AGS Therapeutics.
“Our focus is to ensure that AGS’s manufacturing processes are robust, reproducible, and inspection-ready from the outset. This framework allows us to consolidate GMP capabilities internally, and prepare a seamless transition toward our own dedicated manufacturing unit when the time is right.”
— Lila Drittanti, Co-Founder and CSO/COO at AGS Therapeutics.
“The strategic positioning of INITS SMO is strengthened by this new partnership. The concept was designed to accommodate this type of stakeholder. We are delighted to welcome such innovative project and to offer a unique solution allowing AGS to derisk their project while keeping their know-how”.
— Amel Hadri, Founder & CEO of INITS SMO.
By combining AGS’s proprietary MEV platform with INITS’ GMP-qualified operational environment, the collaboration addresses a key bottleneck in advanced drug delivery: access to clinically compliant manufacturing infrastructure for novel delivery systems. The agreement positions AGS to support first-in-human studies, advance regulatory interactions, accelerate partnering discussions, and strengthen credibility with partners and investors as programs move toward the clinic.
This collaboration represents a controlled yet essential step in AGS’s transition from breakthrough science to industrial-grade GMP execution, reinforcing Europe’s role in the emergence of scalable, sustainable delivery technologies for next-generation therapeutics.
About AGS Therapeutics
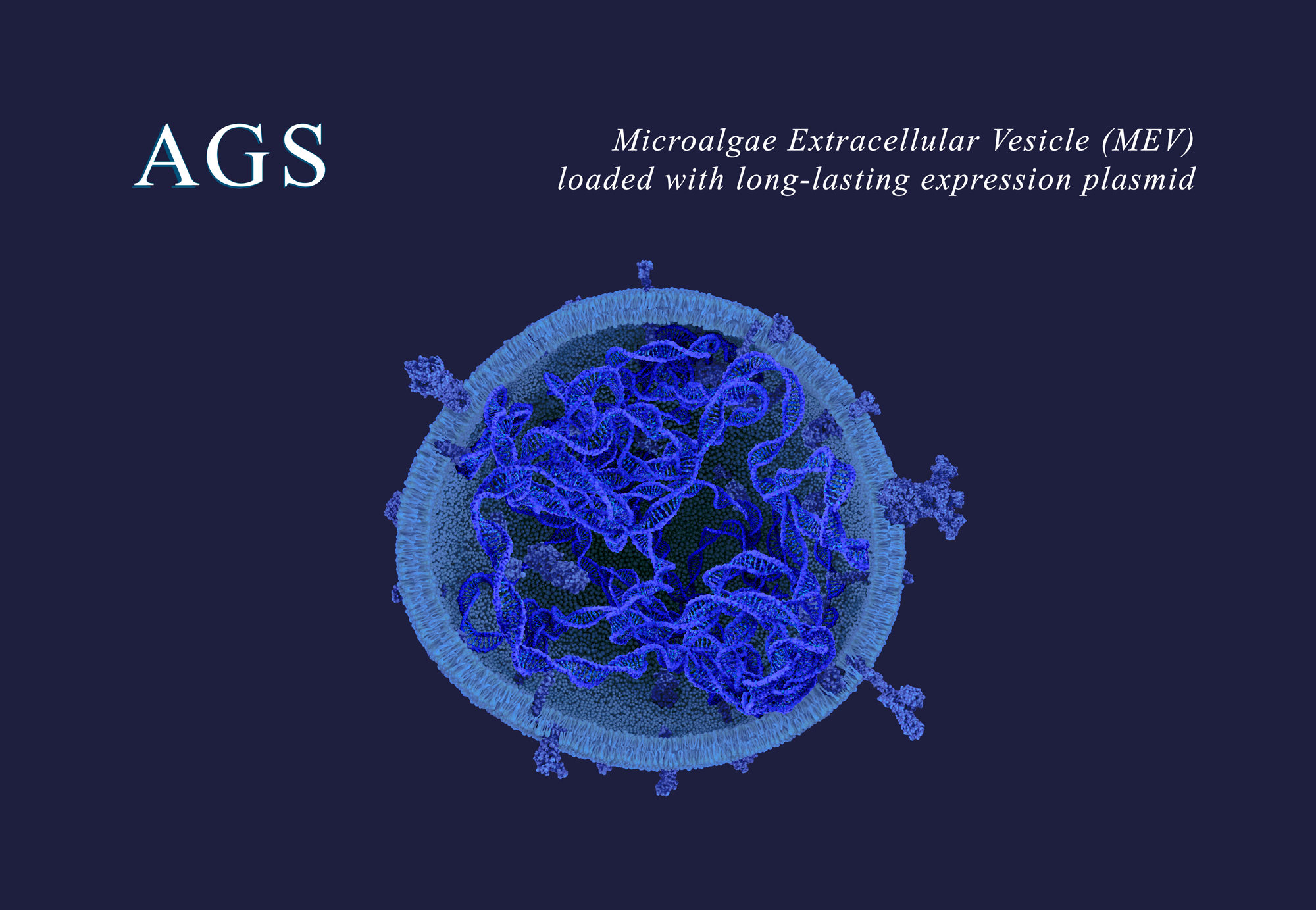
AGS Therapeutics (Evry, France) is pioneering the use of microalgae extracellular vesicles (MEVs) as a universal delivery system for innovative therapeutics, vaccines, and gene therapies. AGS has demonstrated that MEVs are a safe, targeted, and highly versatile delivery platform for mRNA, siRNA, DNA oligonucleotides, plasmids, proteins, and peptides relevant to a wide range of human diseases. AGS-M, the company’s CDMO subsidiary, manufactures MEVs to support AGS’s own research as well as collaborative programs with partner companies. The company’s MEVs are derived from Chlorella, a two-billion-year-old single- cell alga classified by the FDA as GRAS (Generally Recognized as Safe) for consumption as a food supplement. MEVs can be manufactured at scale through eco-friendly and cost-efficient processes. Through strategic collaborations and a commitment to scientific excellence, AGS aims to transform the drug delivery landscape and improve patient outcomes worldwide. For more information, visit www.ags-tx.com and www.ags-m.com.
About INITS
Founded in 2013 and headquartered in Montpellier, France, INITS is a service company supporting biotech companies throughout their pharmaceutical development journey.
INITS has over 12 years of experience and has supported more than 250 clients in France and internationally. With a strong expertise in biologics (cells, genes, EVs, antibodies, proteins...), small and large molecules, and a team of more than 40 experts, INITS covers all pharmaceutical development challenges, including CMC, Regulatory, Clinical Supply, Quality Assurance, and Audits.
For several years, INITS has been developing a disruptive project: INITS SMO, a shared bioproduction unit to be operational from Q2 2026. This unit will allow biotech companies to maintain control over their process by producing themselves their Drug Substance batches while benefiting from INITS’ support (Regulatory, QA, QC, Supply, Fill & Finish, Packaging, Product release and Clinical supply). INITS SMO will also provide Drug Product manufacturing service either from Drug Substance produced by its clients or elsewhere.
For more information about INITS, visit https://inits.fr.
Forward looking statement
This announcement may include predictions, estimates or other information that might be considered forward-looking. While these forward-looking statements represent our current judgment on what the future holds, they are subject to risks and uncertainties that could cause actual results to differ materially. You are cautioned not to place undue reliance on these forward-looking statements, which reflect our opinions only as of the date of this communication.
Media Contacts
AGS Therapeutics
Marie-Hélène Leopold | AGS Therapeutics | +33 (0)6 07 16 55 01 | mhl@ags-tx.com
Ana Vega | Markets & Listing | +33 (0)6 88 57 05 77 | av@markets-listing.com
INITS SMO
Christelle Rochon | Head of Strategic Partnerships & BD | c.rochon@inits.fr