AGS Therapeutics Sets New Standard in Sustainable Manufacturing
PARIS, FRANCE, June 27th, 2025 _ AGS Therapeutics, a pioneering biotech company advancing nextgeneration drug delivery, today unveiled its sustainability statement reaffirming its sustainability commitment.
SUSTAINABILITY COMMITMENT
“Our mission is to transform biomanufacturing from the ground up,” said Dr. Manuel Vega, CEO and co-founder of AGS Therapeutics. “At AGS, we believe sustainability and affordability are not features. They’re our foundations.”
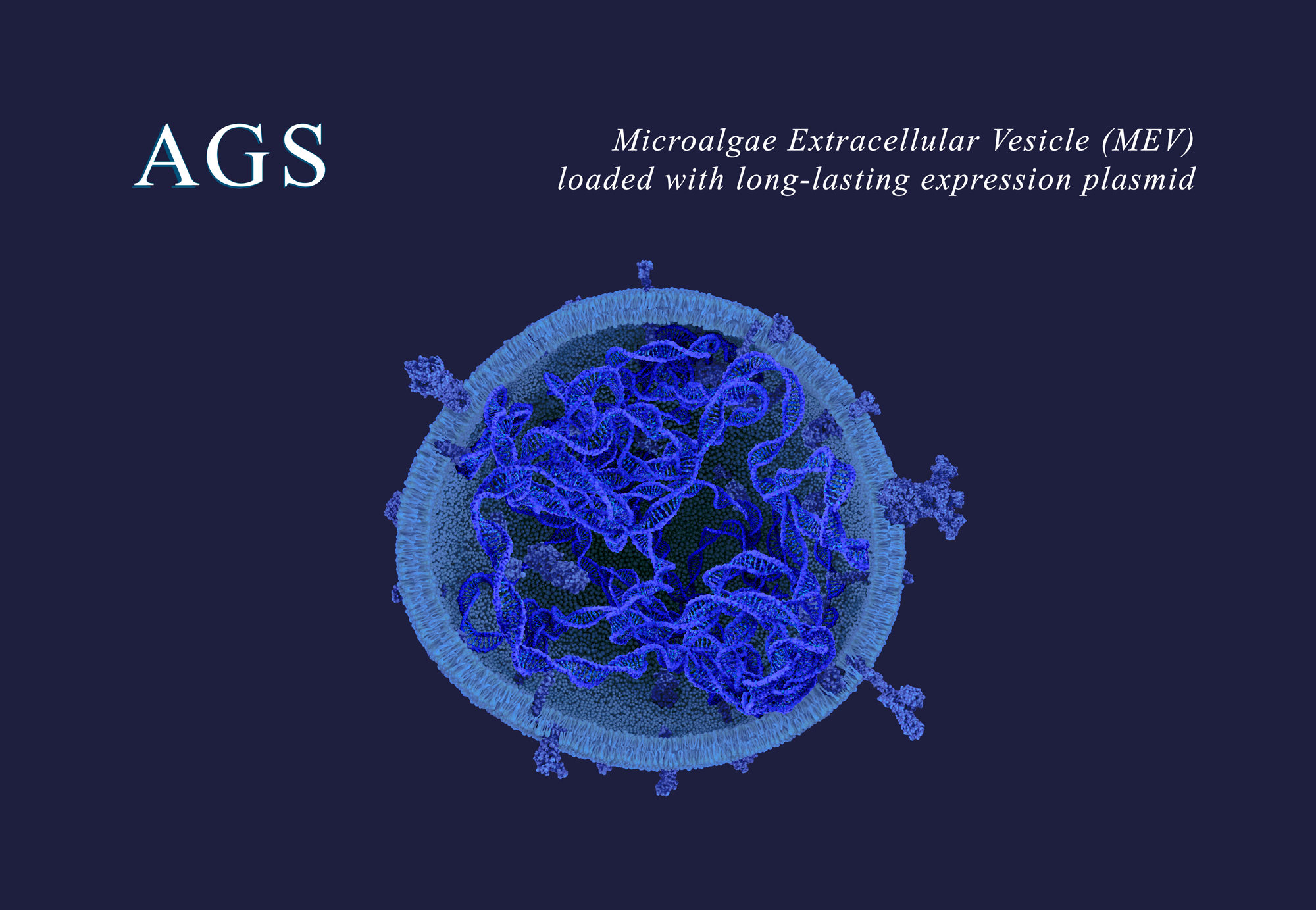
At the heart of AGS’s platform technology are Microalgae Extracellular Vesicles (MEVs) – naturally derived, non-viral, and loadable carriers capable of delivering therapeutic payloads (nucleic acids and proteins) or vaccines with exceptional biocompatibility. AGS’s biomanufacturing platform relies on light, water, and salts to produce advanced biologics through microalgae. Unlike more conventional delivery systems, MEVs and MEV-based therapies are manufactured without animal cells, or harsh solvents or chemicals with minimal resource extraction and high environmental impact. Additionally, MEVs manufacturing is cost-effective and thus holds the potential for a significant social impact in terms of equitable access to advanced and innovative therapies. A simple yet revolutionary biological principle that rests on the sustainability of its fundamental elements.
With an IP-protected platform technology, a growing team, and a roadmap toward GMP-ready production, AGS is positioned to lead a new generation of sustainable biologics. “We envision a future where greener, safer and smarter drug delivery is not the exception – but the standard,” added Dr. Vega.
Learn more about how AGS is building the future of sustainable biotechnology at www.ags-m.com

SUSTAINABILITY STATEMENT
A PARADIGM SHIFT IN SUSTAINABLE BIOTECHNOLOGY IN THE PHARMACEUTICAL INDUSTRY
Our approach represents a paradigm shift that reimagines the pharmaceutical industry through the lens of nature’s principles. Traditional approaches often rely on heavily engineered systems. This reliance on artificial technologies introduces inefficiencies, escalates costs, produces side effects on patients, and exacerbates sustainability challenges. By replacing most of that complexity with the inherent efficiency of natural processes, we are transforming how therapies are developed and delivered. This shift will eventually redefine the infrastructure, culture, processes, and tools that underpin the industry, enabling a future where healthcare is not only simpler and more effective but also seamlessly aligned with global sustainability goals, without compromising on quality.
At AGS (AGS therapeutics, www.ags-tx.com, and its manufacturing subsidiary, AGS-M, www.ags-m.com), we view biotechnology in the pharmaceutical industry as a holistic approach encompassing drug development, manufacturing, distribution, drug administration, and delivery. As such, in developing our technology, we aim to streamline all stages of this lifecycle, ensuring that therapies are not only effective and efficient but also accessible to patients and healthcare providers worldwide.
Aligned with this vision, our sustainability efforts target the environmental, social, and economic dimensions of healthcare. We are focused on reducing resource consumption, minimizing waste, and adopting eco-responsible practices across our operations and our partners. Equally important, we prioritize health equity by creating cost-effective solutions, ensuring that advanced therapies are within reach for all. Through these initiatives, we aim to foster a healthcare ecosystem that is equitable, sustainable, and resilient for future generations.
At the heart of this transformation are extracellular vesicles from microalgae (MEVs), a universal drug delivery system for human therapeutics, vaccines, and gene therapies. These MEVs harness a two-billion-year-old natural technology, refined by evolution, to provide unparalleled efficiency and safety. By leveraging the innate stability, biocompatibility, and scalability of microalgae-derived vesicles, we can address complex healthcare challenges while reducing reliance on synthetic, resource-intensive, and waste-generating processes. This innovation epitomizes our commitment to blending nature’s principles with cutting-edge science to deliver therapies that are effective, accessible, and aligned with the imperatives of sustainability.
THE BROKEN STATE OF THE PHARMACEUTICAL INDUSTRY
Today’s drug production demands vast amounts of resources, including energy, water, organic solvents, synthetic materials, animal-derived components, and sometimes viral vectors. These inputs create substantial environmental impacts, from increased greenhouse gas emissions to the generation of hazardous waste. The widespread use of single-use plastics and complex, expensive infrastructure further compounds these challenges. Moreover, the pharmaceutical industry’s resource intensity poses risks to ecosystems and places a significant burden on waste management systems.
Equity and accessibility remain critical issues. Many existing therapies are invasive, or highly invasive, making them difficult to administer and less likely to be followed by patients. The use of drug delivery systems like adeno-associated viruses (AAVs) and lipid nanoparticles (LNPs) introduces additional complications. While AAVs offer targeted delivery, they carry risks of immune responses and high costs, i.e. inequity, concerns. LNPs face challenges related to poor biodistribution, toxicity, the massive use of hazardous organic solvents, and the need for complex storage and transport logistics. These systemic issues underscore the need for innovative solutions that balance efficacy, accessibility, and sustainability.
Despite these significant shortcomings, the pharmaceutical industry largely accepts the status quo, perpetuating inertia and an undue reliance on established mental models, which, while useful, are inherently flawed. Yet they dominate industry practices due to familiarity and entrenched investment. This persistence leaves little room for critical questioning. To break this cycle, targeted innovation and substantial funding are urgently needed to redefine the pharmaceutical industry for the better.
THE UNREASONABLE EFFECTIVENESS OF MEVS AS A UNIVERSAL DRUG DELIVERY SYSTEM
MEVs are produced using Chlorella, a genetically stable and renewable microalgae species generally recognized as safe (GRAS). The manufacturing process is resource-efficient, requiring only light, salts, and water. This eliminates the need for synthetic components, organic solvents, and animal-derived materials, significantly reducing water usage and the overall environmental footprint. The process involves the production of general-purpose MEVs from wild-type (non-GMO) algae, where waste algae and water can be recycled, further enhancing sustainability. These MEVs are versatile and can be stored long-term, ready for use with any therapeutic, vaccine, or gene therapy payload, streamlining production and distribution.
MEV production supports high-volume scalability, minimal waste generation, and compatibility with various payloads, including mRNA, siRNA, proteins, peptides, and small molecules. The Chemistry, Manufacturing, and Controls (CMC) process for MEVs is common and shared across all payloads of the same payload type, and the platform is advancing toward a single, unified CMC process for all payload types. This soon-to-be GMP-compliant innovation simplifies drug development and regulatory approval, significantly reducing both timelines and costs.
Therapies developed with MEVs are stable for long-term storage and can be delivered through non-invasive methods such as pills or sprays, reducing the reliance on single-use plastics and disposable materials. Formulation, distribution, routes of administration, biodistribution, uptake, and delivery depend entirely on the MEV itself, which remains consistent across all payloads. This quality simplifies and unifies the entire therapeutic approach, making it more efficient and scalable.
From a sustainability perspective, MEVs excel across environmental, social, and economic dimensions. They minimize resource consumption, eliminate hazardous inputs, and simplify logistical requirements, reducing overall costs. Socially, their ease of administration and affordability expand access to advanced therapies, particularly in underserved populations. Economically, MEVs streamline manufacturing and supply chain processes, enabling scalability in emerging markets without imposing significant financial burdens.
Unlike AAVs, MEVs are non-viral, eliminating risks of immune responses. Compared to LNPs, MEVs are naturally stable, biocompatible, and free from toxicity risks, simplifying storage and handling. Furthermore, MEVs surpass mammalian EVs by offering consistent quality at scale. In all cases, MEVs avoid ethical concerns tied to animal-derived materials. These attributes make MEVs a superior alternative for the delivery of therapeutics, vaccines, and gene therapies.
LEADERSHIP, DIVERSITY, AND SUSTAINABILITY IN ACTION
AGS places a strong emphasis on leadership that reflects gender equality and ethnic diversity. Women currently occupy a significant percentage of management roles, with all current positions, except for the current CEO, and future leadership appointments intentionally guided by these principles. This commitment fosters a culture of innovation, inclusivity, and collaboration.
To strengthen its sustainability efforts, AGS is establishing internal guidelines and will soon appoint a dedicated VP of Sustainability. This role will ensure transparency and align initiatives across drug development, manufacturing, and partnerships with both internal and global sustainability goals, embedding sustainability at every level by design.
INTELLECTUAL PROPERTY AND LONG-TERM ECONOMIC VALUE
AGS secures its position as a leader in MEV technology with a robust portfolio of nearly ten patents, and growing, and extensive proprietary know-how. This intellectual property not only protects the company’s innovations but also facilitates their widespread adoption across the industry. By offering a sustainable alternative to synthetic, viral, and animal-based delivery systems, AGS enables the healthcare sector to transition toward more ethical and environmentally responsible practices, underscoring its long-term value to both the industry and society.
About AGS Therapeutics
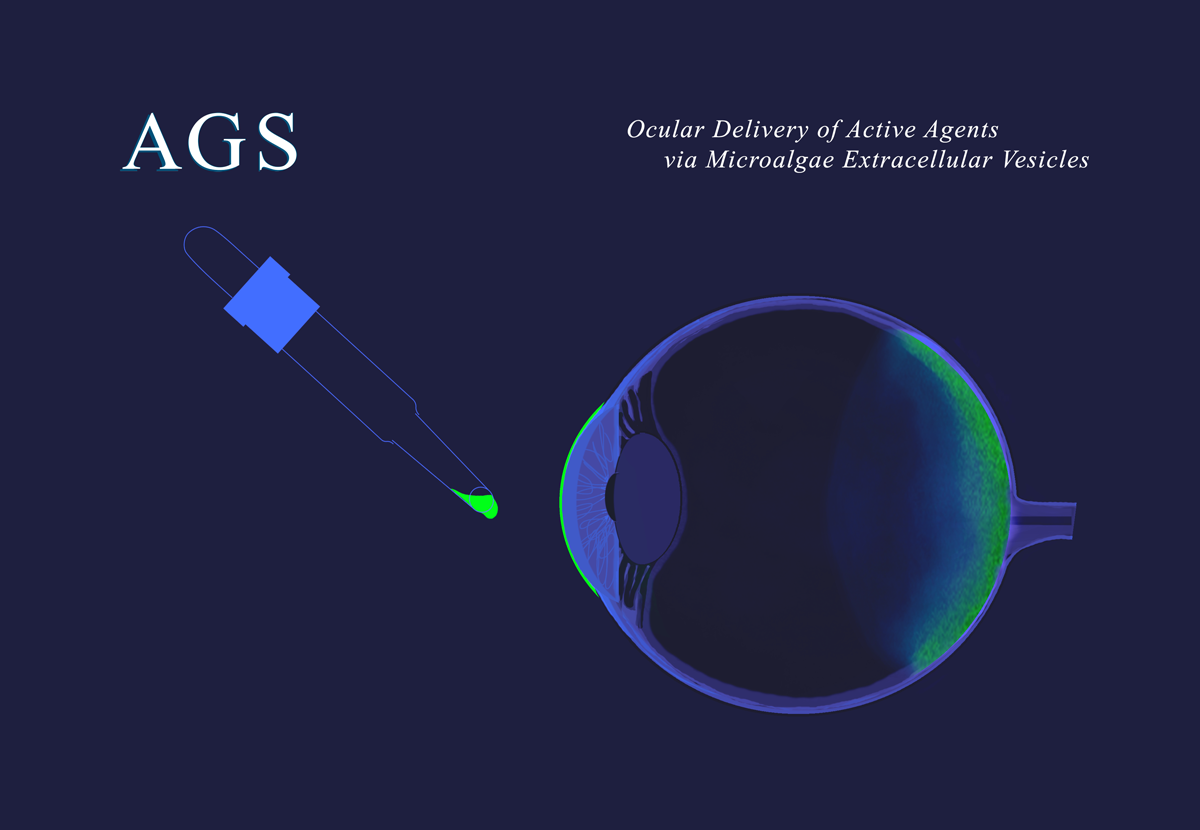
AGS Therapeutics, based in Paris and at Genopole (www.genopole.fr), Evry, France, is a biotech company pioneering the use of microalgae extracellular vesicles (MEVs) as a universal delivery system for innovative therapeutics, vaccines and gene therapies. AGS has shown MEVs to be a safe, targeted and highly versatile delivery system for mRNA, siRNA, DNA oligos, plasmids, proteins, and peptides relevant to a broad range of human diseases. AGS's programs include AGS-1010 and AGS-2010. AGS-1010 carries an anti-angiogenesis agent loaded into MEVs and is intended for the treatment of wAMD. AGS-1010 can be delivered to the back of the eye-by-eye drops (drop instillation), which differentiates it from all products (in the market or in development, for the treatment of wAMD). AGS-2010 carries a modulator of TLR-9 activity, loaded into MEVs, and is intended for the treatment of IBD. AGS-2010 is delivered directly to the intestinal epithelial cells following the oral administration of the loaded MEVs. AGS-2010 differentiates from current treatments of IBD by the highly targeted delivery to the site of action (the intestine) through the intestinal lumen, thus avoiding the systemic route. AGS-M, the company’s CDMO subsidiary, produces the MEVs needed to support R&D at AGS and for companies partnering with AGS. AGS’s MEVs are derived from Chlorella, a two-billion-year-old single-cell algae, labelled by the FDA as GRAS for consumption as a food supplement. AGS’s MEVs are easy to manufacture in large quantities with processes that are sustainable, straightforward (simple), scalable, resource-effective, and cost-effective. Through strategic partnerships and a commitment to scientific excellence, the company aims to challenge the delivery landscape and improve the lives of patients across the globe. For more information, visit www.ags-tx.com and www.ags-m.com.
Forward-Looking Statement
This announcement may include predictions, estimates or other information that might be considered forward-looking. While these forward-looking statements represent our current judgment on the future, they are subject to risks and uncertainties that could cause actual results to differ materially. Readers cautioned not to place undue reliance on these forward-looking statements, which reflect our opinions only as of the date of this communication.
Contacts
Marie-Hélène Leopold | AGS Therapeutics | +33 (0)6 07 16 55 01 | mhl@ags-tx.com
Ana Vega | Markets & Listing | +33 (0)6 88 57 05 77 | av@markets-listing.com